Class 10th Science Biology Experiment 8 Study of different properties of acetic acid (ethanoic acid) Answer or Solutions.
Aim : To study the following properties of acetic (ethanoic) acid :(1) Odour (2) Solubility in water (3) Effect on litmus (4) Reaction with sodium bicarbonate.
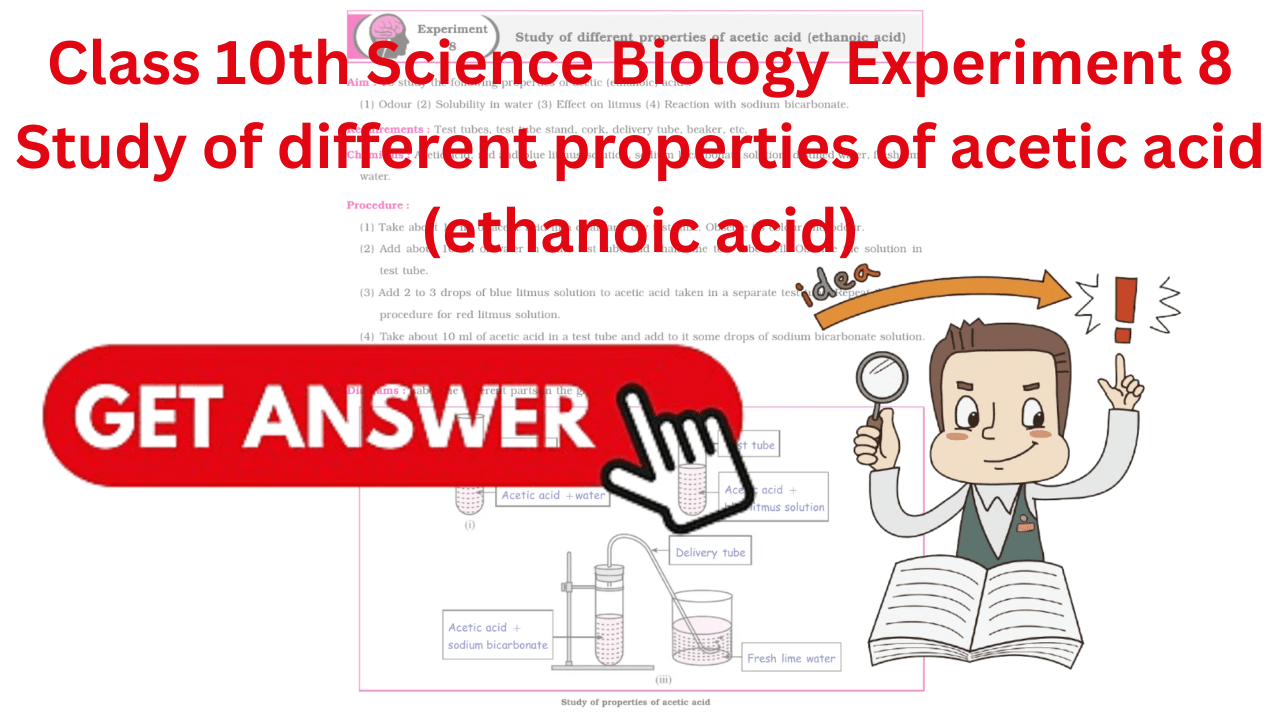
Requirements : Test tubes, test tube stand, cork, delivery tube, beaker, etc.
Chemicals : Acetic acid, red and blue litmus solution, sodium bicarbonate solution, distilled water, fresh lime water.
Procedure :
- Take about 10 ml of acetic acid in a clean and dry test tube. Observe its colour and odour.
- Add about 10 ml of water in same test tube and shake the test tube well. Observe the solution intest tube.
- Add 2 to 3 drops of blue litmus solution to acetic acid taken in a separate test tube. Repeat the sameprocedure for red litmus solution.
- Take about 10 ml of acetic acid in a test tube and add to it some drops of sodium bicarbonate solution.Pass the gas through fresh lime water.
Diagrams : Label the different parts in the given diagram.


Multiple Choice Questions:
Choose the correct alternative and write its letter (A), (B), (C), (D) in the box :
1. Ethanoic ( Acetic ) acid .
(A) is odourless (B) has a smell of ammonia (C) has smell of rotten eggs (D) has a vinegar smell
Answer: D
2. Acetic ( Ethanoic ) acid .
(A) turns red litmus blue (B) has pungent odour (C) is red in colour (D) is odourless
Answer: B
3. When sodium bicarbonate solution is added to dilute acetic acid .
(A) CO2 gas is evolved (B) a solid settles at the bottom (C) the mixture becomes warm (D) the colour of the mixture becomes yellow
Answer: A
4. is liberated when acetic acid reacts with sodium metal.
(A) H 2 (B) O2 (C) CO2 (D) NH 3
Answer: A
5. Ethanoic acid which solidifies below 290K is known as .
(A) ice (B) solid acetic acid (C) glacial acetic acid (D) ethyl acetate
Answer: C