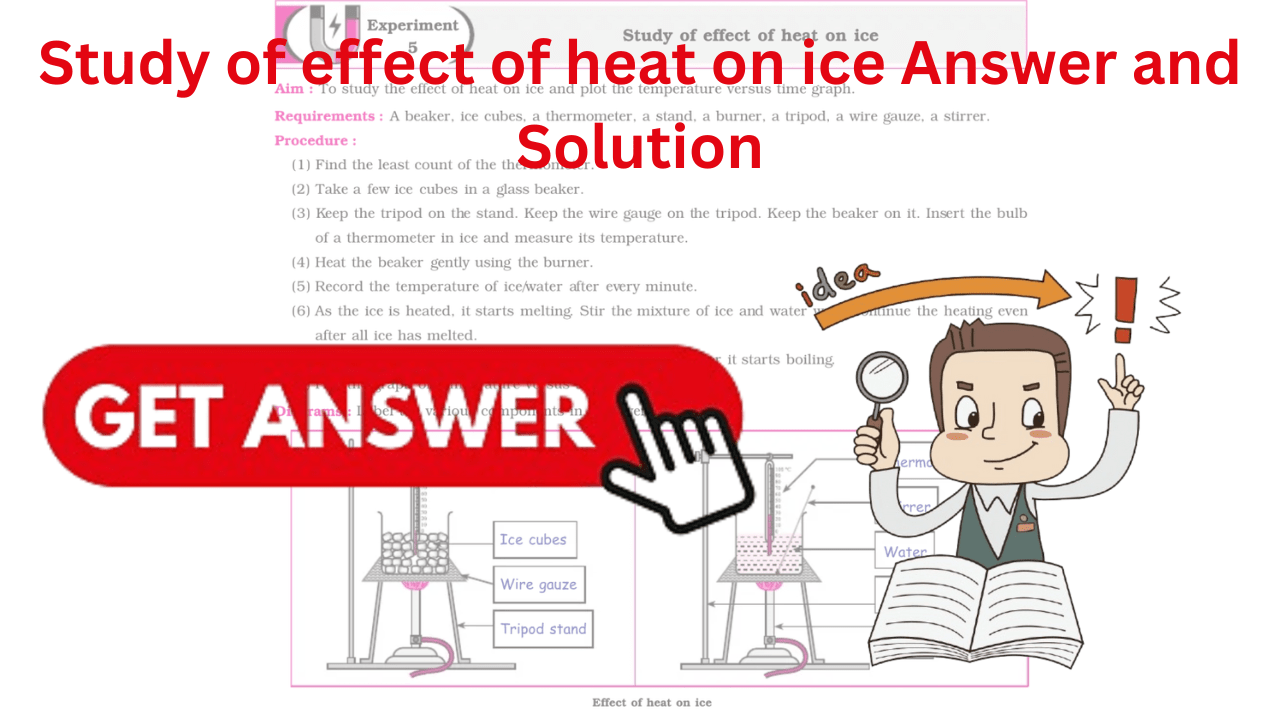
Standard 10th Science Experiment 5 Answer Solutions: Study of effect of heat on ice Answer and Solution.
Aim :
To study the effect of heat on ice and plot the temperature versus time graph.
Requirements :
A beaker, ice cubes, a thermometer, a stand, a burner, a tripod, a wire gauze, a stirrer.
Procedure :
(1) Find the least count of the thermometer. (2) Take a few ice cubes in a glass beaker. (3) Keep the tripod on the stand. Keep the wire gauge on the tripod. Keep the beaker on it. Insert the bulbof a thermometer in ice and measure its temperature. (4) Heat the beaker gently using the burner. (5) Record the temperature of ice/water after every minute. (6) As the ice is heated, it starts melting. Stir the mixture of ice and water well. Continue the heating evenafter all ice has melted. (7) Continue to record the temperature of the water even after it starts boiling. (8) Plot the graph of temperature versus time.
Diagrams :
Label the various components in the given diagram.




Multiple Choice Questions:
Choose the correct alternative and write its letter (A), (B), (C), (D) in the box :
1. To observe the reaction of water on quick lime, .
(A) quick lime is added to water in a test tube (B) a lot of water is added to quick lime (C) a few drops of water are added to quick lime (D) none of these
Answer: C
2. When crystals of Ferrous sulphate are strongly heated, the residue obtained is .
(A) red in colour (B) blue in colour (C) green in colour (D) colourless
Answer: A
3. When Barium chloride solution is added to Sodium sulphate solution, .
(A) blue precipitate is formed (B) white precipitate is formed (C) green precipitate is formed (D) no reaction takes place
Answer: B
4. The reaction of iron nails with copper sulphate solution is an example of .
(A) combination reaction (B) decomposition reaction (C) displacement reaction (D) double displacement reaction
Answer: C
5. Reddish brown deposit formed on iron nails kept in a solution of copper sulphate is .
(A) Cu2O (B) Cu (C) CuO (D) CuS
Answer: B