Standard 10th Science Experiment 1 Answer Solutions: Identification of halide ions (Cl – , Br – and I – ions) Answer Solutions. Experiment 1: Identification of halide ions (Cl – , Br – and I – ions)
Aim : To identify the presence of chloride, bromide and iodide ions from the given salt solutions.
Requirements : Test tubes, test tube stand, dropper, beaker, etc.
Chemicals : Silver nitrate solution (AgNO3), solutions of potassium chloride, potassium bromide and potassiumiodide.
Procedure :
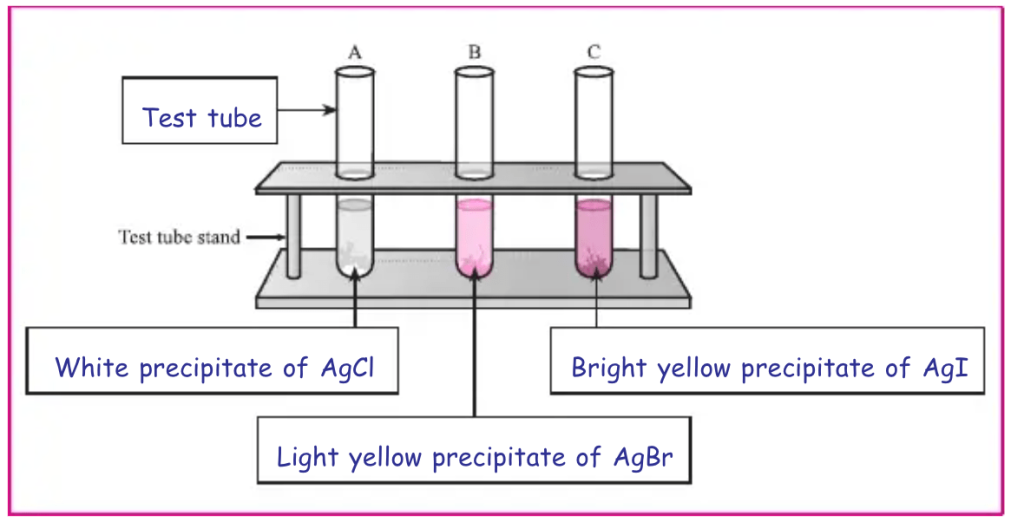
- Take three test tubes and label them as A, B and C.
- Take about 5 ml of solutions of potassium chloride in test tube A, potassium bromide in test tube Band potassium iodide in test tube C.
- Add 4 to 5 drops of silver nitrate solution in each test tube and stir it.
- Observe the colour of precipitate formed in each test tube and record your observations.
- Depending upon the colour of the precipitate, identify the halide present in the solution.
Diagram : Label the various components in the given diagram.
Identification of halide ions (Cl –, Br– and I – ions)
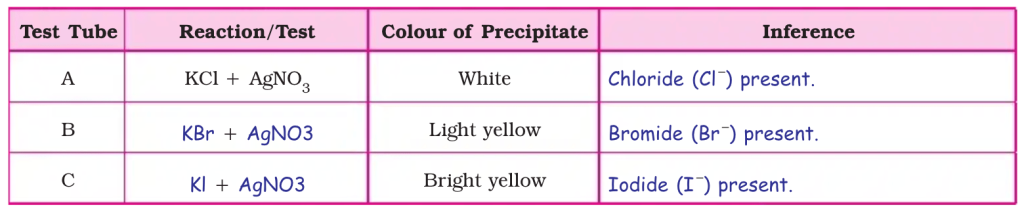
Observation table :
Chemical reactions :


Inferences :
(1) Halide ions (Cl –, Br–, I –) are precipitated in all the three reactions of above experiments. (2) In the above experiment, depending on the colour of the precipitates, presence of Cl –, Br– and I – in the given solutions are confirmed. (3) Elements of halogen group in the periodic table show similar properties.
Multiple Choice Questions:
•
Choose the correct alternative and write its letter (A), (B), (C), (D) in the box :
1. The number of electrons in the outermost shell of halogens is .
(A) 1 (B) 2 (C) 3 (D) 7
Answer: D
2. is in solid state at room temperature.
(A) Fluorine (B) Iodine (C) Chlorine (D) Bromine
Answer: B
3. Which of the following substances is the strongest reducing agent?
(A) Cl2 (B) Cl–ion (C) Br (D) Br–ion
Answer: D