Standard 10th Science Experiment 10 Answer Solutions: Arranging the metals according to decreasing order of their reactivity Answer Solutions
Aim :
To study the reactions of the metals Al, Zn, Fe and Cu with the solutions of salts Al2(SO4)3, ZnSO4, FeSO4, CuSO4 and to arrange these metals in decreasing order of their reactivity.
Requirements :
Test tubes, Test tube stand, distilled water, sandpaper, forceps, the metals Aluminium, Zinc,Iron and Copper, solutions of the salts Al2(SO4)3, ZnSO4, FeSO4, CuSO4.
Procedure :
(1) Take four 50 ml beakers and label them as Aluminium sulphate, Zinc sulphate, Ferrous sulphate andCopper sulphate.
(2) Clean all the metallic pieces using sandpaper and cut them into small pieces.
(3) Add 10 ml of solution of Al2(SO4)3, ZnSO4, FeSO4 and CuSO4 in the labelled beakers respectively.
(4) Add two pieces of aluminium metal into beaker containing ZnSO4, FeSO4 and CuSO4 solutions.
(5) Keep the beakers undisturbed for about 20 minutes.
(6) After about 20 minutes, note the change in colour of solutions or any other change.
(7) Repeat the similar procedure by adding – Zinc pieces to Al2(SO4)3, FeSO4 and CuSO4 solutions, Ironpieces to Al2(SO4)3, ZnSO4 and CuSO4 solutions, Copper pieces to Al2(SO4)3, ZnSO4 and FeSO4 solutions.
(8) Record your observations.
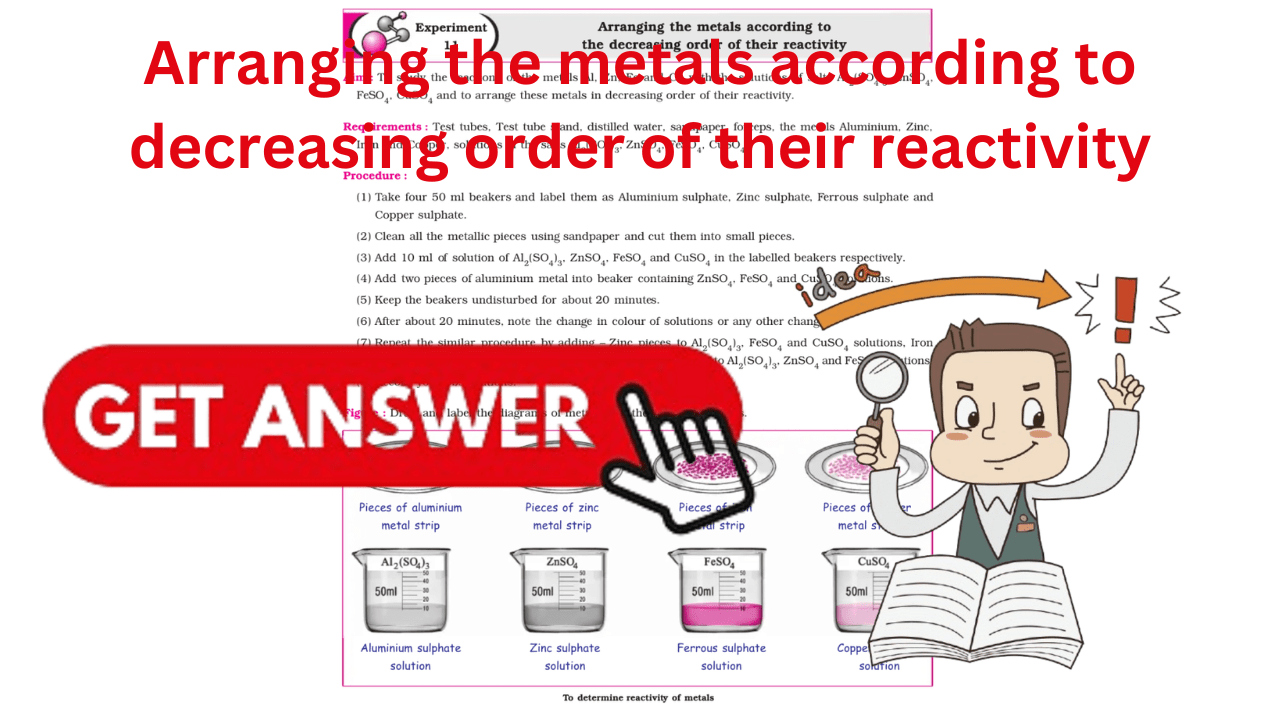
Figure :
Draw and label the diagrams of metals with the solutions of salts.



Multiple Choice Questions
Choose the correct alternative and write its letter (A), (B), (C), (D) in the box :
1)To show that zinc is more reactive than copper, the correct procedure is to .
(A) prepare copper sulphate solution and dip zinc strip in it
(B) prepare zinc sulphate solution and dip copper strip in it
(C) heat together zinc and copper strips
(D) add dil. nitric acid to both the strips
Answer: A
2. A solution of Al2(SO4)3 in water is not clear. It is due to .
(A) impurities present in water
(B) hydrolysis of Al2 (SO4)3 in water
(C) impurities present in Al2 (SO4) 3
(D) none of these
Answer: D
3. Iron is .
(A) more reactive than zinc
(B) more reactive than aluminium
(C) less reactive than copper
(D) less reactive than aluminium
Answer: D